Course Planning and Assessments
As part of my graduate program, I created a portfolio focused on online assessments for a fictional planned course. This work was created for educational purposes only, for the University of Cincinnati.
The content was generated under, and templates provided by, Dr. Karen Lankisch.
Course Planning and Alignment
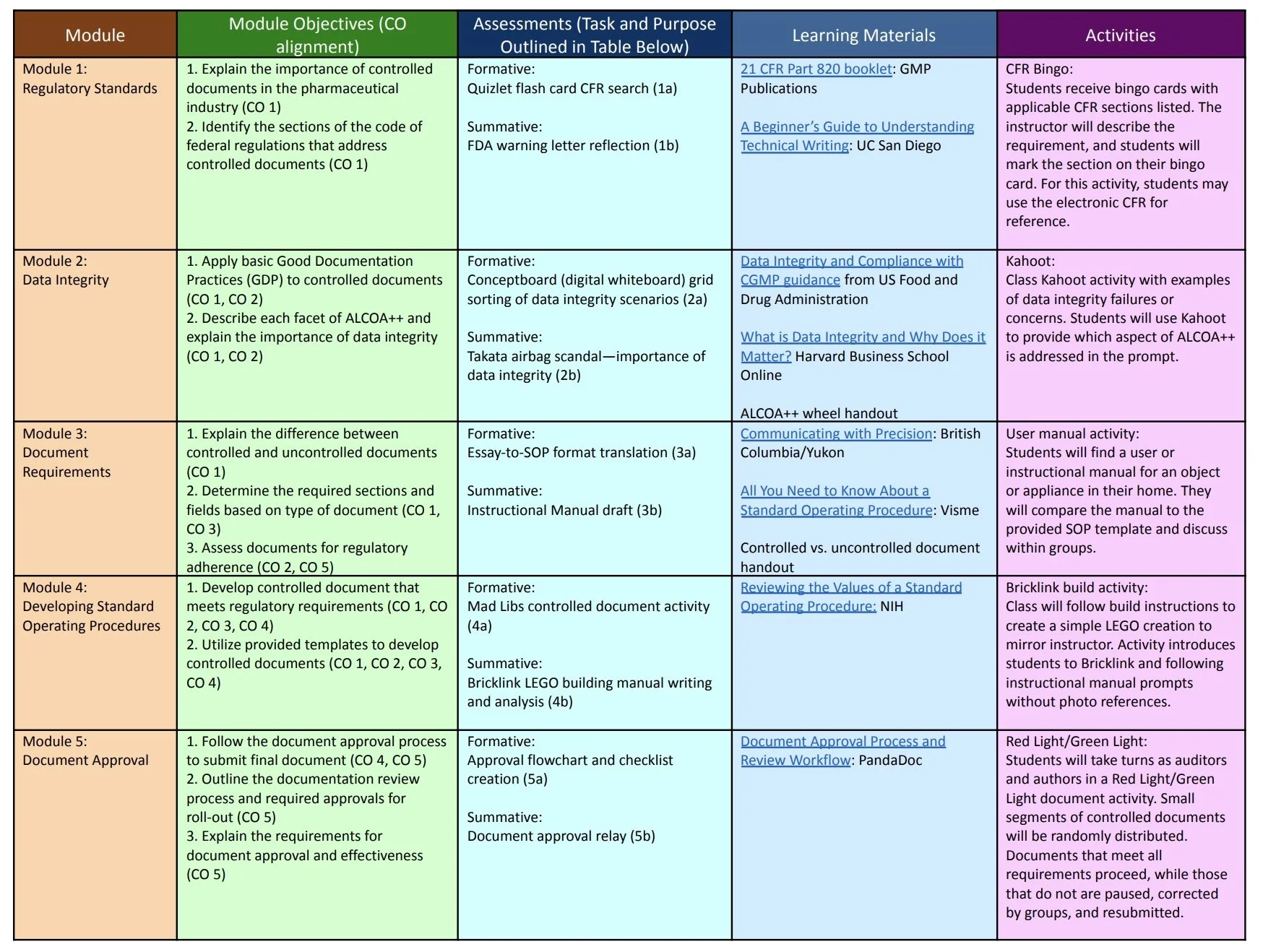
I was tasked with planning my own course, with a focus on creating relevant learning assessments. The assessments and learning activities must align with my created course objectives. The purpose of the project was to include a variety of assessment options for an online course. I included both formative and summative assessments, tying each one to its related objective.
Full descriptions of each assessment, the required task, the purpose, and scaffolding can be viewed here:
https://docs.google.com/document/d/1byLdvydg2fbU1S8pBfpIvhVRLlzX9h0U/edit?usp=sharing&ouid=106607670686514014793&rtpof=true&sd=true
Course Title: Authoring Controlled Documentation
Course Description: This course will prepare quality specialists for authoring controlled documentation for a pharmaceutical organization. These documents include standard operating procedures, work instructions, and policies that require adherence to regulatory standards.
Course Objectives (CO):
List regulatory requirements for controlled documents by section
Apply data integrity principles to electronic documentation
Utilize standardized templates and regulatory language to write compliant documentation
Develop standard operating procedures and other controlled documents for use in the pharmaceutical industry
Explain each step of the document review and approval process, and outline their importance
Assessment Rubrics
Formative Assessment
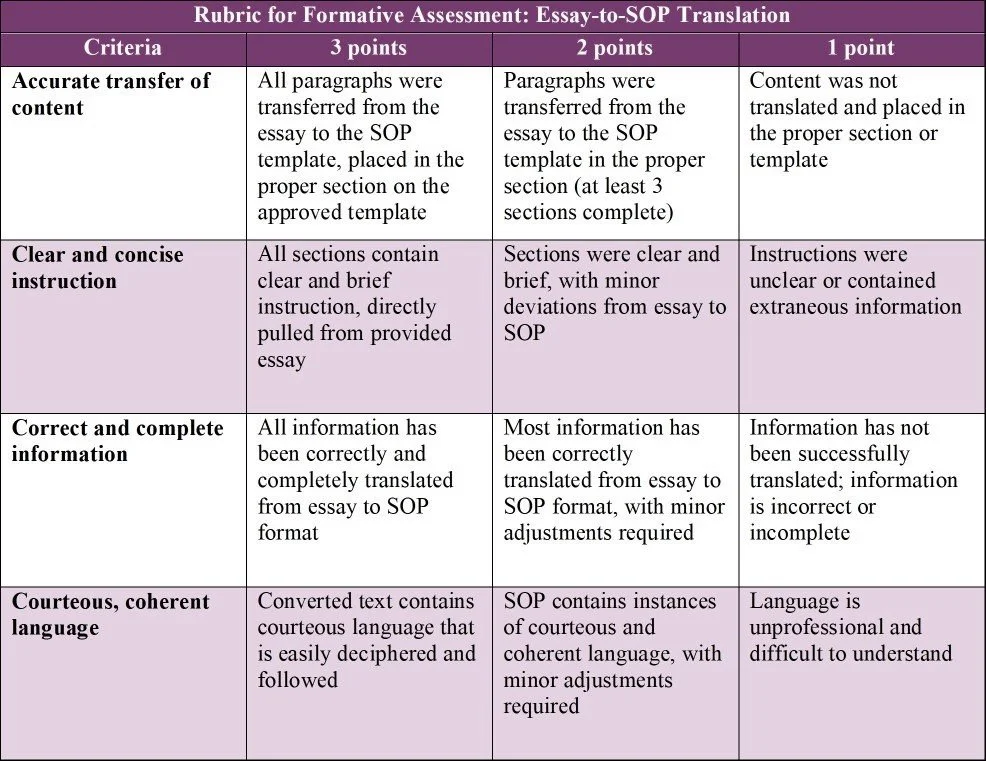
For one formative assessment, the students will work in pairs to assemble a pre-written document into a structured standard operating procedure (SOP). The students will receive a copy of a deconstructed SOP, written in an essay format. They will also receive an SOP template with only headings filled in. Together, the students will convert each paragraph of the essay into a cohesive standard operating procedure, ensuring all five sections (purpose, scope, roles and responsibilities, abbreviations and definitions, and procedure content) are present and complete. All information must be transferred from essay format to the standard operating procedure template. The instruction must clear, coherent, concise, concrete, correct, complete, and courteous.
The purpose of this activity is to provide students with hands-on practice in converting standard text to clear, concise steps and instructions. This assignment aligns with:
Course Objective 1: Understand controlled documentation requirements and principles
Course Objective 3: Utilize standardized templates and regulatory language to write compliant documentation
Summative Assessment
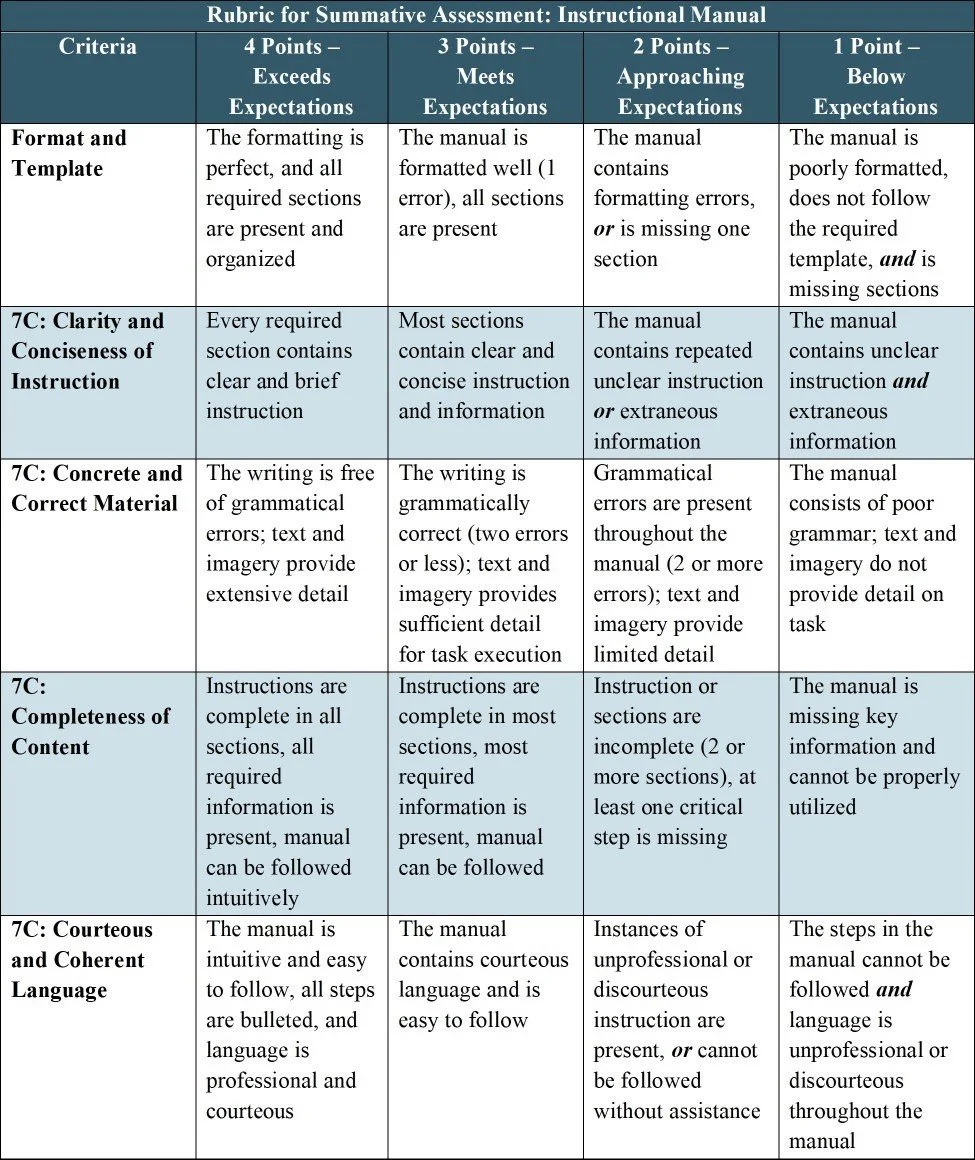
One summative assessment tasks the students with developing their own controlled document. Each student will select an object of their choosing from their home, classroom, or workspace. Using the provided template, each student will create an instructional manual for the object. At a minimum, the manual must include a diagram of the object, an explanation of its purpose, instructions on its use, and safety considerations. The manual must follow the Seven Cs of technical writing: clear, coherent, concise, concrete, correct, complete, and courteous.
The purpose of this assessment is to evaluate the student’s ability to write a controlled document and follow technical requirements. Over the course of the module, students learned about SOP templates, the Seven Cs, data integrity, and Good Documentation Practices (GDP). This assessment aligns with:
Course Objective 2: Apply data integrity principles to electronic documentation
Course Objective 3: Utilize standardized templates and regulatory language to write compliant documentation
Course Objective 4: Develop standard operating procedures and other controlled documents for use in the pharmaceutical industry